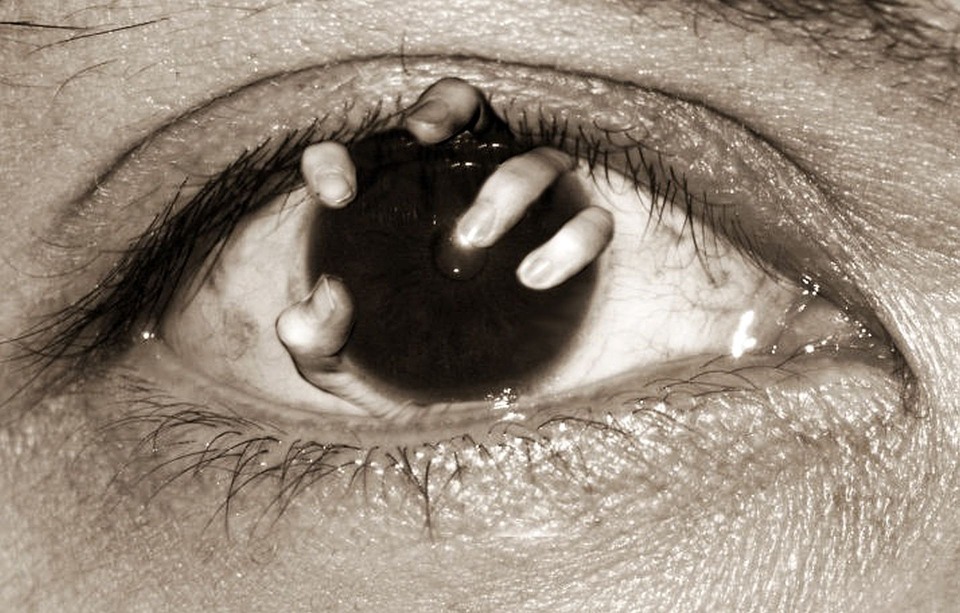
Providing hope to thousands of people suffering from hereditary blindness, advisers for the US Food and Drug Administration just gave a critical recommendation to a new type of treatment. Using gene therapy, the treatment that researchers called Luxturna is intended to reverse the damage caused by a rare form of blindness that is inherited.
The treatment was developed by Spark Therapeutics Inc. and targets Leber congenital amaurosis, which is a hereditary form of blindness, Reuters reports. Now, it’s worth pointing out that the recommendation does not automatically mean approval. The members of the advisory panel simply gave their thoughts on the matter, but the FDA itself is not obligated to follow their recommendations.
If it does get approved, however, it would be the first gene therapy treatment of its kind to get the coveted badge. It could also pave the way for future approvals of similar treatments, which could accelerate the development of cures for some of the world’s deadliest diseases and birth defects.
As for what swayed the panel to provide their recommendations, it was apparently due to the presentation that Spark provided as well as the testimonies of the patients who tried the treatment. Many of them described going out to see the stars for the first time as well as getting to experience life along with their friends.
As with all things that go through tons of red tape, however, approval is likely to take time. Spark can apparently expect to get a response from the FDA sometime around January, Business Insider reports. After that, it will still take a while for the drug to actually hit the market and get to the hands of those who need it.
Then again, patients already waited this long to get the drug. A few months won’t likely make much of a difference.



 Tabletop particle accelerator could transform medicine and materials science
Tabletop particle accelerator could transform medicine and materials science  Neuren Pharmaceuticals Surges on U.S. Patent Win for Rare Disorder Drug
Neuren Pharmaceuticals Surges on U.S. Patent Win for Rare Disorder Drug  NASA Astronauts Wilmore and Williams Recover After Boeing Starliner Delay
NASA Astronauts Wilmore and Williams Recover After Boeing Starliner Delay  Cogent Biosciences Soars 120% on Breakthrough Phase 3 Results for Bezuclastinib in GIST Treatment
Cogent Biosciences Soars 120% on Breakthrough Phase 3 Results for Bezuclastinib in GIST Treatment  FDA Pilot Program Eases Rules for Nicotine Pouch Makers
FDA Pilot Program Eases Rules for Nicotine Pouch Makers  CDC Vaccine Review Sparks Controversy Over Thimerosal Study Citation
CDC Vaccine Review Sparks Controversy Over Thimerosal Study Citation  Neuralink Plans High-Volume Brain Implant Production and Fully Automated Surgery by 2026
Neuralink Plans High-Volume Brain Implant Production and Fully Automated Surgery by 2026  Trump Administration to Launch Autism Initiatives Targeting Acetaminophen Use and New Treatment Options
Trump Administration to Launch Autism Initiatives Targeting Acetaminophen Use and New Treatment Options  Trump Signs Executive Order to Boost AI Research in Childhood Cancer
Trump Signs Executive Order to Boost AI Research in Childhood Cancer  Trump and Merck KGaA Partner to Slash IVF Drug Costs and Expand Fertility Coverage
Trump and Merck KGaA Partner to Slash IVF Drug Costs and Expand Fertility Coverage  NASA Faces Major Workforce Reduction as 20% of Employees Prepare to Leave
NASA Faces Major Workforce Reduction as 20% of Employees Prepare to Leave  Blue Origin’s New Glenn Achieves Breakthrough Success With First NASA Mission
Blue Origin’s New Glenn Achieves Breakthrough Success With First NASA Mission