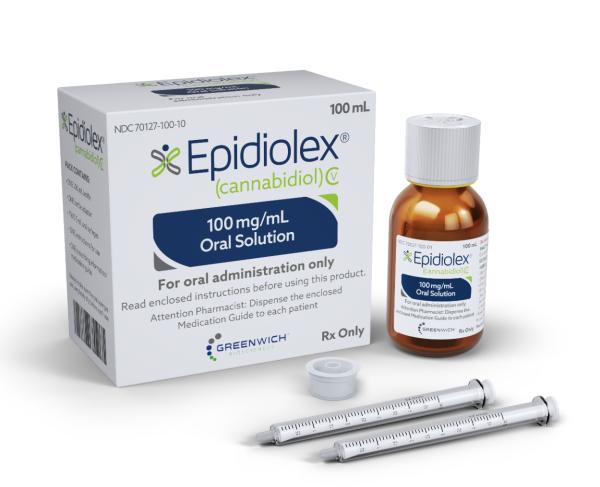
The US Food and Drug Administration (FDA) is planning to regulate the use of the cannabis compound CBD in food and supplements after weighing the evidence on the compound's safety.
The FDA will decide whether regulating legal cannabis will require new agency rules or new legislation from Congress.
Cannabis products, excluding Epidiolex, are illegal at the federal level in the US, although some states allow their use.
According to Patrick Cournoyer, who heads the FDA office developing the agency's cannabis strategy, the FDA will ascertain if CBD can be safely eaten every day for a long period or during pregnancy amid concerns about future fertility.
.



 Supreme Court Signals Doubts Over Trump’s Bid to Fire Fed Governor Lisa Cook
Supreme Court Signals Doubts Over Trump’s Bid to Fire Fed Governor Lisa Cook  Once Upon a Farm Raises Nearly $198 Million in IPO, Valued at Over $724 Million
Once Upon a Farm Raises Nearly $198 Million in IPO, Valued at Over $724 Million  Trump Endorses Japan’s Sanae Takaichi Ahead of Crucial Election Amid Market and China Tensions
Trump Endorses Japan’s Sanae Takaichi Ahead of Crucial Election Amid Market and China Tensions  American Airlines CEO to Meet Pilots Union Amid Storm Response and Financial Concerns
American Airlines CEO to Meet Pilots Union Amid Storm Response and Financial Concerns  Meta Faces Lawsuit Over Alleged Approval of AI Chatbots Allowing Sexual Interactions With Minors
Meta Faces Lawsuit Over Alleged Approval of AI Chatbots Allowing Sexual Interactions With Minors  FDA Targets Hims & Hers Over $49 Weight-Loss Pill, Raising Legal and Safety Concerns
FDA Targets Hims & Hers Over $49 Weight-Loss Pill, Raising Legal and Safety Concerns  Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing
Novo Nordisk Launches Once-Daily Wegovy Pill in U.S. at Competitive Pricing  California Jury Awards $40 Million in Johnson & Johnson Talc Cancer Lawsuit
California Jury Awards $40 Million in Johnson & Johnson Talc Cancer Lawsuit  Global Markets Slide as AI, Crypto, and Precious Metals Face Heightened Volatility
Global Markets Slide as AI, Crypto, and Precious Metals Face Heightened Volatility  Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies
Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies  Citigroup Faces Lawsuit Over Alleged Sexual Harassment by Top Wealth Executive
Citigroup Faces Lawsuit Over Alleged Sexual Harassment by Top Wealth Executive  Trump Family Files $10 Billion Lawsuit Over IRS Tax Disclosure
Trump Family Files $10 Billion Lawsuit Over IRS Tax Disclosure  Trump Lifts 25% Tariff on Indian Goods in Strategic U.S.–India Trade and Energy Deal
Trump Lifts 25% Tariff on Indian Goods in Strategic U.S.–India Trade and Energy Deal  Panama Supreme Court Voids CK Hutchison Port Concessions, Raising Geopolitical and Trade Concerns
Panama Supreme Court Voids CK Hutchison Port Concessions, Raising Geopolitical and Trade Concerns