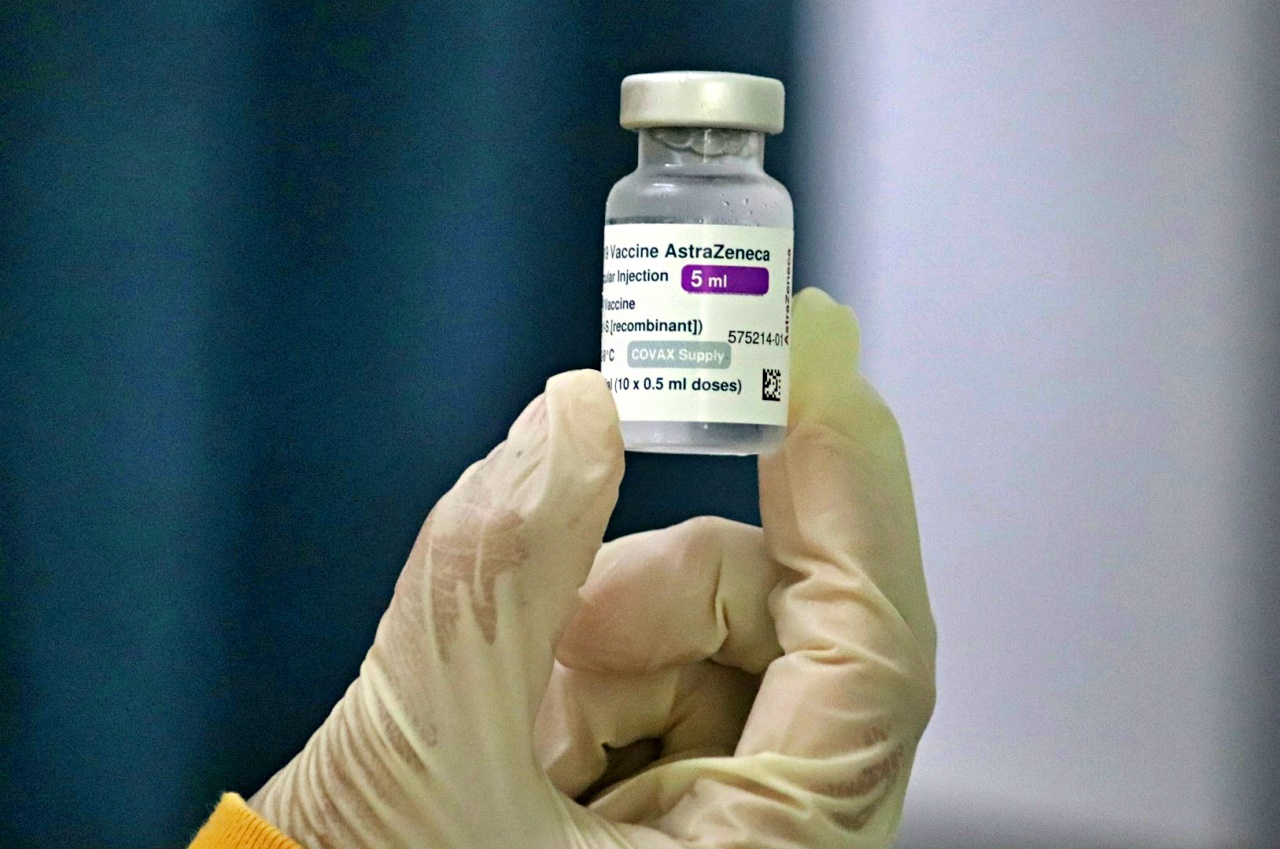
AstraZeneca plc, a British-Swedish pharmaceutical and biotechnology company, is reportedly withdrawing its COVID-19 vaccine worldwide. The company made the decision months after admitting in court there is a rare side effect.
But then again, according to Channel News Asia, AstraZeneca did not say this was the reason for the pullout of the vaccines. Rather, the company said it was due to “commercial reasons.”
Taking Back the COVID-19 Vaccines Years After Release
AstraZeneca said on Tuesday, May 7, the withdrawal of its vaccines around the world is already underway. It stressed that this is due to a "surplus of available updated vaccines" since the pandemic.
Moreover, the pharmaceutical and biotech firm announced it would also retract the vaccine Vaxzevria's marketing authorizations in Europe. AstraZeneca added that it is no longer producing its COVID-19 vaccine, so supply to the market has also stopped.
“As multiple, variant Covid-19 vaccines have since been developed there is a surplus of available updated vaccines,” the company’s spokesman said in a statement. “This has led to a decline in demand for Vaxzevria, which is no longer being manufactured or supplied.”
Reported Side Effects of Vaxzevria
AstraZeneca reportedly admitted in court documents that the vaccine has side effects like low platelet counts and blood clots. British newspapers stated that based on legal documents, the company's vaccine may cause TTS, but in very rare cases. World Health Organization (WHO), said this condition called Thrombosis with Thrombocytopenia Syndrome is a serious and life-threatening negative effect.
“It is admitted that the AZ vaccine can, in very rare cases, cause TTS,” AstraZeneca said in court documents in February. “The causal mechanism is not known.”
UK’s The Independent reported that AstraZeneca’s admission came after it was sued in the U.K. The class action lawsuits alleged that the company’s vaccine caused deaths and severe injuries, and claimants are seeking damages up to £100 million for around 50 supposed victims.
Photo by: Mufid Majnun/Unsplash



 Baidu Approves $5 Billion Share Buyback and Plans First-Ever Dividend in 2026
Baidu Approves $5 Billion Share Buyback and Plans First-Ever Dividend in 2026  Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market
Viking Therapeutics Sees Growing Strategic Interest in $150 Billion Weight-Loss Drug Market  Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies
Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies  TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans
TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans  Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links
Tencent Shares Slide After WeChat Restricts YuanBao AI Promotional Links  Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing
Weight-Loss Drug Ads Take Over the Super Bowl as Pharma Embraces Direct-to-Consumer Marketing  Instagram Outage Disrupts Thousands of U.S. Users
Instagram Outage Disrupts Thousands of U.S. Users  Rio Tinto Shares Hit Record High After Ending Glencore Merger Talks
Rio Tinto Shares Hit Record High After Ending Glencore Merger Talks  OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering
OpenAI Expands Enterprise AI Strategy With Major Hiring Push Ahead of New Business Offering  Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch
Global PC Makers Eye Chinese Memory Chip Suppliers Amid Ongoing Supply Crunch  Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies
Missouri Judge Dismisses Lawsuit Challenging Starbucks’ Diversity and Inclusion Policies  FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program
FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program  Sanofi Reports Positive Late-Stage Results for Amlitelimab in Eczema Treatment
Sanofi Reports Positive Late-Stage Results for Amlitelimab in Eczema Treatment  Royalty Pharma Stock Rises After Acquiring Full Evrysdi Royalty Rights from PTC Therapeutics
Royalty Pharma Stock Rises After Acquiring Full Evrysdi Royalty Rights from PTC Therapeutics  Ford and Geely Explore Strategic Manufacturing Partnership in Europe
Ford and Geely Explore Strategic Manufacturing Partnership in Europe  TrumpRx Website Launches to Offer Discounted Prescription Drugs for Cash-Paying Americans
TrumpRx Website Launches to Offer Discounted Prescription Drugs for Cash-Paying Americans