new anti-osteoporosis drug has just been approved for use in the UK. The drug, called abaloparatide, is licensed for post-menopausal women with osteoporosis and a very high risk of fractures. Abaloparatide’s approval could benefit more than 14,000 women in the UK who are at serious risk of bone fracture.
Osteoporosis affects about 3.8 million people in the UK. The disorder causes bones to become fragile due to low bone mass and deterioration in bone structure. This makes them more likely to break.
The risk of developing osteoporosis increases with age – and is especially common in women after the menopause. This is because their oestrogen levels decrease. Oestrogen is protective to bones, as it slows down the natural bone breakdown. Around half of women over 50 will experience a bone fracture.
Until now, most commonly used osteoporosis treatments were anti-resportive drugs. These work by slowing down or preventing the natural bone loss that is accelerated after the menopause.
But abaloparatide works differently. It actually promotes the formation of new bone. It does this by mimicking a natural hormone called parathyroid hormone-related protein, or PTHrP. This hormone plays a role in bone development and calcium regulation.
Abaloparatide selectively activates the parathyroid hormone 1 receptor found on the surface of our bone-building cells (osteoblasts). This promotes bone formation, which in turn increases bone density and reduces fracture risk in people with osteoporosis.
In one research study that tested the drug’s efficacy, abaloparatide reduced the risk of spine fractures by 88% compared to a placebo. The risk of other major fractures (such as hip, spine, wrist and upper arm) were reduced by 69%.
After an initial period of 18 months, where the drug was compared to placebo, the study was then extended by 24 months and both groups were then given the commonly-used anti-resorptive treatment, alendronic acid. This drug only slows down bone loss.

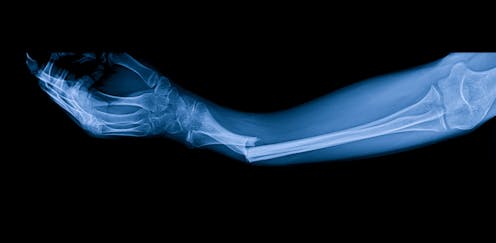
The drug was shown to be very effective at preventing fractures. Elnur/ Shutterstock
The group that received abaloparatide followed by alendronic acid had an 84% lower risk of having a new spine fracture compared to the group that had received the placebo first followed by alendronic acid.
The study also compared abaloparatide to teriparatide, another bone-building drug in use in the UK since 2002. The two drugs were equally effective at preventing fractures. This finding has also been confirmed by a 2022 study which compared real-world use of the two drugs. Abaloparatide was licensed in the US in 2017 and has been used by many patients there.
Preventing bone fractures
Abaloparatide comes in a pre-filled pen that delivers the drug directly under the skin. Patients will need to take daily injections, usually in the abdomen. Patients can only be on the drug for 18 months. This is because the bone-building effect is greatest in the first year – then it wears off. After 18 months of abaloparatide, patients will be prescribed an anti-resorptive agent to help maintain the newly formed bone.
Abaloparatide is usually well tolerated. The most common side-effects are nausea, dizziness, headaches and heart palpitations. In some rare instances, abaloparatide may increase blood calcium levels. This could cause problems with heart, muscle and nerve function.
Abaloparatide is also free from many of the side-effects caused by anti-resportive drugs – such as osteonecrosis of the jaw. This is a very rare condition which causes injuries in the mouth to take longer to heal than normal.
Abaloparatide is just the latest bone-building drug licensed for use in the UK. Teriparatide and romosozumab are already available to patients with very high risk of bone fracture. And like with these other bone-building agents, abaloparatide has only been approved for post-menopausal patients with osteoporosis who are at very high risk of fracture.
Fractures can have severe consequences – including chronic pain, loss of independence and even increased risk of death. Abaloparatide is a new addition to the osteoporosis-fighting arsenal which will greatly benefit people with a very high risk of fracture.



 RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash
RFK Jr. Overhauls Federal Autism Panel, Sparking Medical Community Backlash  Novo Nordisk Stock Surges After FDA Approves Wegovy Pill for Weight Loss
Novo Nordisk Stock Surges After FDA Approves Wegovy Pill for Weight Loss  FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program
FDA Fast-Tracks Approval of Altria’s on! PLUS Nicotine Pouches Under New Pilot Program  Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccines Portfolio
Sanofi to Acquire Dynavax in $2.2 Billion Deal to Strengthen Vaccines Portfolio  Innovent Biologics Shares Rally on New Eli Lilly Oncology and Immunology Deal
Innovent Biologics Shares Rally on New Eli Lilly Oncology and Immunology Deal  AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study
AstraZeneca’s LATIFY Phase III Trial of Ceralasertib Misses Primary Endpoint in Lung Cancer Study  TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans
TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans  Eli Lilly and Novo Nordisk Battle for India’s Fast-Growing Obesity Drug Market
Eli Lilly and Novo Nordisk Battle for India’s Fast-Growing Obesity Drug Market  Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan
Federal Appeals Court Blocks Trump-Era Hospital Drug Rebate Plan