An Osaka University research team has concluded that the world's first clinical trial, transplanting corneal tissues derived from induced pluripotent stem cells, or iPS cells, was safe and effective.
According to the team at Osaka University led by Professor Koji Nishida, none of the four almost-blind patients had rejection or tumorigenicity of the transplanted cells, and all of their symptoms improved.
Three of the patients had improved vision, with one going from 0.15 to 0.7, according to the researchers. The iPS cells were developed by Shinya Yamanaka of Kyoto University, who was awarded the Nobel Prize in Physiology or Medicine in 2012 for his work. They can grow into any form of bodily tissue.
In 2023, a clinical trial will be conducted with the goal of putting the treatment into practice within the next three to four years.
The new approach is expected to address concerns such as transplant rejection and a chronic scarcity of corneal donors. As of the end of March 2021, some 1,700 patients in Japan were waiting for corneal donations.
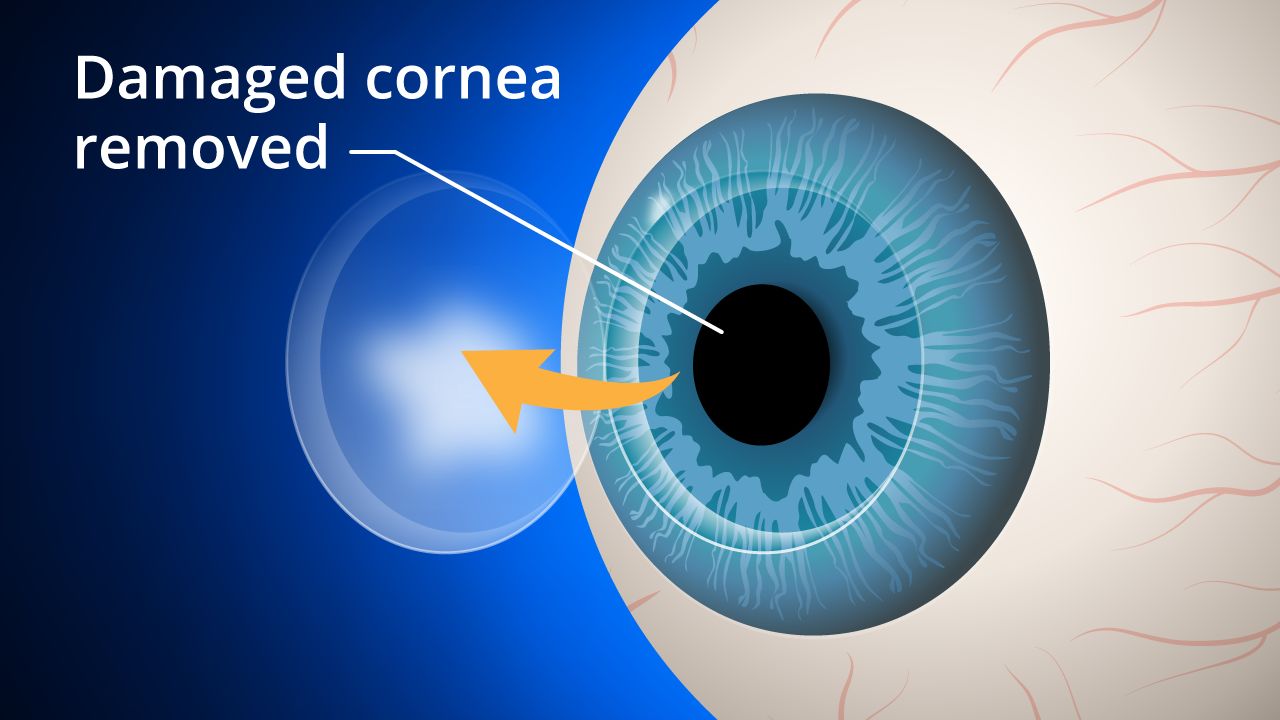
To construct 0.05-millimeter thick sheet-like corneal tissues for the clinical trials, researchers cultured corneal cells from another individual's iPS cells housed at Kyoto University.
The transplants were conducted on four patients in their 30s to 70s who had corneal epithelial stem cell deficit, a disorder characterized by the loss of cells in the eye that make the cornea, between July 2019 and December 2020.
Other than transplants, there is no effective treatment for the illness, which can cause vision loss and deterioration.



 U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors
U.S. Vaccine Policy Shifts Under RFK Jr. Create Uncertainty for Pharma and Investors  South Korea’s Weak Won Struggles as Retail Investors Pour Money Into U.S. Stocks
South Korea’s Weak Won Struggles as Retail Investors Pour Money Into U.S. Stocks  NASA and Roscosmos Chiefs Meet in Florida to Discuss Moon and ISS Cooperation
NASA and Roscosmos Chiefs Meet in Florida to Discuss Moon and ISS Cooperation  Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies
Novo Nordisk and Eli Lilly Cut Obesity Drug Prices in China as Competition Intensifies  Thailand Inflation Remains Negative for 10th Straight Month in January
Thailand Inflation Remains Negative for 10th Straight Month in January  Oil Prices Slide on US-Iran Talks, Dollar Strength and Profit-Taking Pressure
Oil Prices Slide on US-Iran Talks, Dollar Strength and Profit-Taking Pressure  Asian Markets Slip as AI Spending Fears Shake Tech, Wall Street Futures Rebound
Asian Markets Slip as AI Spending Fears Shake Tech, Wall Street Futures Rebound  Trump Backs Review of U.S. Childhood Vaccine Schedule After Hepatitis B Policy Change
Trump Backs Review of U.S. Childhood Vaccine Schedule After Hepatitis B Policy Change  Trump Endorses Japan’s Sanae Takaichi Ahead of Crucial Election Amid Market and China Tensions
Trump Endorses Japan’s Sanae Takaichi Ahead of Crucial Election Amid Market and China Tensions  FDA Lifts REMS Requirement for CAR-T Cell Cancer Therapies
FDA Lifts REMS Requirement for CAR-T Cell Cancer Therapies  Trump Signs Executive Order to Boost AI Research in Childhood Cancer
Trump Signs Executive Order to Boost AI Research in Childhood Cancer  Global Markets Slide as AI, Crypto, and Precious Metals Face Heightened Volatility
Global Markets Slide as AI, Crypto, and Precious Metals Face Heightened Volatility  U.S.-India Trade Framework Signals Major Shift in Tariffs, Energy, and Supply Chains
U.S.-India Trade Framework Signals Major Shift in Tariffs, Energy, and Supply Chains  TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans
TrumpRx.gov Highlights GLP-1 Drug Discounts but Offers Limited Savings for Most Americans  Silver Prices Plunge in Asian Trade as Dollar Strength Triggers Fresh Precious Metals Sell-Off
Silver Prices Plunge in Asian Trade as Dollar Strength Triggers Fresh Precious Metals Sell-Off  U.S. and Rwanda Sign $228 Million Health Partnership to Boost Self-Reliance
U.S. and Rwanda Sign $228 Million Health Partnership to Boost Self-Reliance